Enroll for Biologics, Biosimilars & Vaccines Regulatory Affairs Online Course
Job Overview
-
Date PostedDecember 20, 2025
-
Location
-
Expiration dateDecember 22, 2025
-
Click to apply:
Job Description
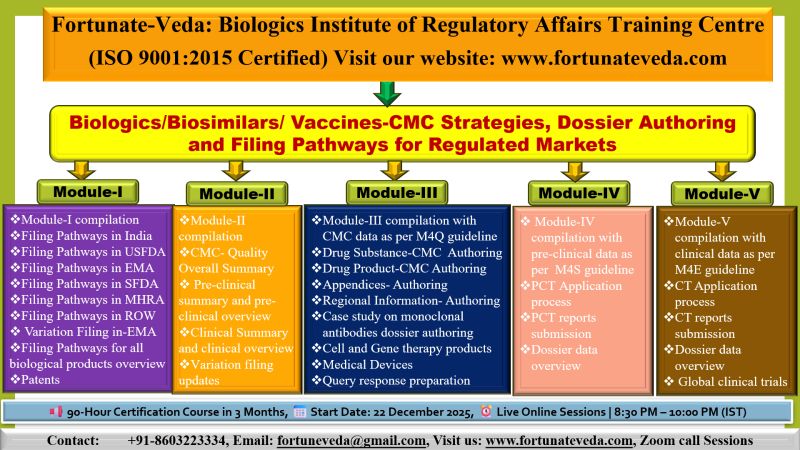
Invitation – Biologics, Biosimilars & Vaccines Regulatory Affairs Online Course
We are delighted to invite you to join our Biologics, Biosimilars & Vaccines Regulatory Affairs Online Course.
Please find attached the detailed course schedule and program benefits for your reference.
✨ Batch Details
📅 Start Date: 22 December 2025
🗓️ Duration: December-2025 to March-2026 (3 months / 90 hours)
⏰ Timings: 08:30 PM – 10:00 PM (IST)
📌 Mode: Live sessions on Zoom
Fee: Rs. 30000 (INR)
2 Free sessions to get glimpse of course and confirm enrollment.
👩💼 Who Can Attend?
This program is ideal for:
Professionals from Pharma, Biologics, Vaccines, Biosimilars, and Cell & Gene Therapy domains
Individuals working in Regulatory Affairs (RA), Quality Assurance (QA), R&D, and Manufacturing
Fresh graduates or professionals aspiring to build a career in Regulatory Affairs
📲 For quick assistance or more details, please connect with us on WhatsApp: +91-8603223334
We look forward to your participation!
Best regards,
Fortunate-Veda
📞
+91-8603223334 | fortuneveda@gmail.com
🌐 www.fortunateveda.com
✉️ fortuneveda@gmail.com
🌐 www.fortunateveda.com